Impact of antiresorptive drugs on dental implant success rate: A systematic review
Article information
Abstract
Purpose
This systematic review aimed to evaluate the impact of antiresorptive drugs on dental implant success rate, and to suggest guidelines for dental implant placement in patients under antiresorptive therapies.
Materials and Methods
An electrical search was conducted in databases such as PubMed and Cochrane, followed by Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines. the review included studies from 2014 march, focusing on various study designs like randomized control studies, cohort studies, and case series.
Results
In a total of 196 articles, based on inclusion criteria, 9 articles were selected. the dental implant success rates across the studies ranged from 36.2% to 100%. a notable association was identified between the dosage and administration route of antiresorptive drugs and dental implant success rates. additionally, presence of dental implant could be potential risk for Medication-Related Osteonecrosis of the Jaw (MRONJ) onset in patients under antiresorptive therapy.
Conclusion
Low dose and oral administration of antiresorptive drugs seem to present a lower risk of dental implant failure. a multidisciplinary approach is important when considering dental implants for patients on antiresorptive therapy. Further research is required to refine clinical guidelines for enhanced patient safety and better outcomes in dental implantology.
Introduction
Antiresorptive drugs, including bisphosphonates and denosumab, are pivotal in bone remodeling and are widely used to treat osteoporosis and various cancer-related conditions [1]. Their role in modulating bone resorption makes them essential in managing disorders linked to bone degradation and loss [2,3]. as more patients receive these medications, oral and maxillofacial surgeons face challenges related to adverse effects, notably medication-related osteonecrosis of the jaw (MRONJ) [4].
MRONJ incidence is strongly correlated with the dosage regimen of antiresorptive drugs [5]. Research shows that cancer patients, who receive doses 12 to 15 times higher than those for osteoporosis, have a significantly increased risk of developing MRONJ [1,6,7]. This dosage-dependent risk complicates the balance between treatment efficacy and safety for healthcare providers.
furthermore, dentoalveolar procedures such as tooth extractions and dental implant placements have been identified as local risk factors for MRONJ, though the specific risk associated with dental implants remains debated. a recent position paper by the american association of oral and maxillofacial surgeons noted that the exact risk in patients on antiresorptive therapy is still uncertain and under investigation [1].
Given the current lack of robust evidence or guidelines regarding the safety of antiresorptive drugs in dental implantology, there is a clear need for updated data and systematic assessments. This review aims to critically evaluate the impact of antiresorptive therapy on dental implant success and failure rates and to propose recommendations that will contribute to safer clinical practices in dental implant procedures [8].
Material and Method
This systematic review was conducted following the PRISMA guidelines [9].
Eligibility criteria
studies published from 2014 onward were considered. eligible designs included randomized controlled trials (RCTs), cohort studies, case-control studies, and case series, each involving at least five patients without co-morbidities undergoing antiresorptive therapy in relation to dental implant surgery. a minimum follow-up period of six months post-surgery was required to ensure sufficient osseointegration, and only full-text publications were included. Studies failing to meet these criteria were excluded.
Information sources
a comprehensive search was performed in electronic databases-PubMed and the cochrane central register of controlled trials-with the search concluding on June 28, 2022.
Search strategy
The search targeted studies published since 2014 using keywords such as antiresorptive drugs, denosumab, bisphosphonate, dental implant placement, implant success rate, and implant failure. The PICO (Patient, Intervention, Comparison, Outcome) framework was employed to construct precise search queries.
Selection process
Two independent reviewers (KMK, KHJ) screened titles and abstracts using EndNote, removing duplicate records. eligible studies then underwent full-text review, with any disagreements resolved by a third reviewer (PYW).
Data collection process
Data extraction was independently performed by two reviewers (KMK, KHJ) using a standardized excel spreadsheet. discrepancies were discussed and resolved with the involvement of a third reviewer (PYW).
Data items
Extracted data included the number of implant successes and failures, patient and implant success rates, implant failure rates, and outcomes related to implant failures. additional pertinent information was also collated.
Study risk of bias assessment
Risk of bias in RCTs was evaluated using Version 2 of the Cochrane risk-of-bias tool, while non-randomized studies were assessed with the ROBINS-I tool to ensure a thorough evaluation of bias across included studies.
Results
The search strategy initially identified 196 articles. after screening, 35 articles were selected for detailed eligibility assessment, and following full-text evaluations, 26 were excluded. ultimately, 9 articles were included in this systematic review (Fig. 1).
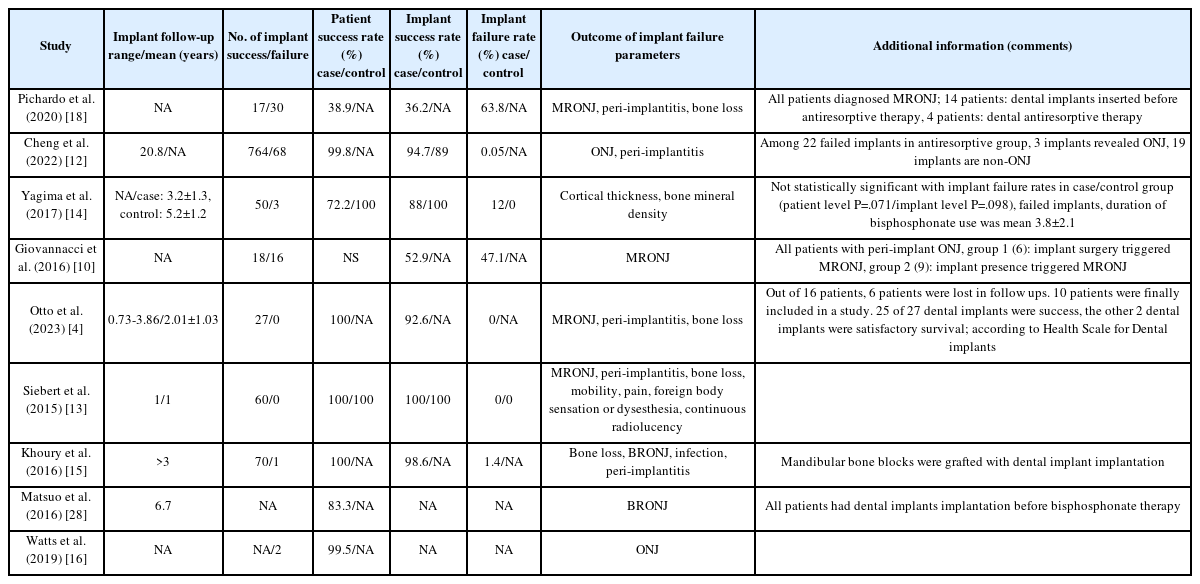
The selected studies comprised one randomized controlled trial (RCT), one prospective cohort study, one case-control study, two case series, and four retrospective cohort studies (Table 1). detailed data on patient demographics, drug regimens, and implant outcomes are provided in Tables 1-3.
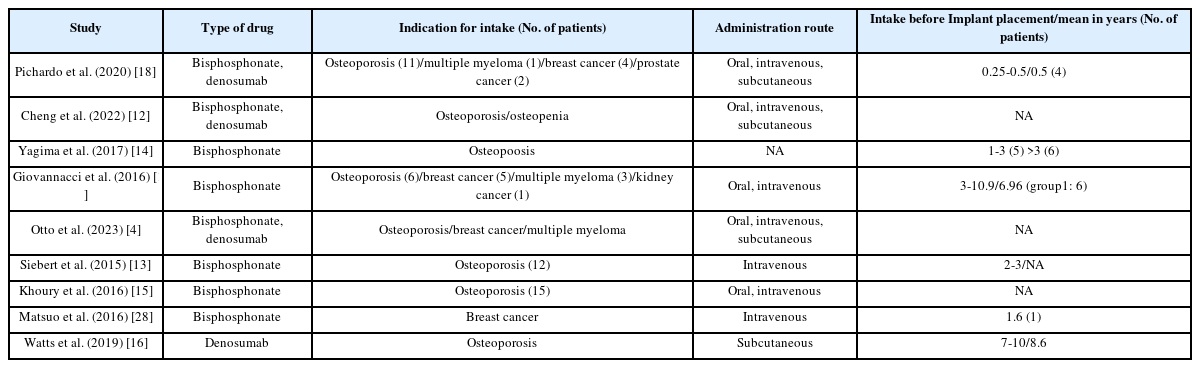
Most studies focused on bisphosphonates or a combination of bisphosphonates and denosumab, with only one study exclusively addressing denosumab. the majority of patients were postmenopausal women undergoing antiresorptive therapy for osteoporosis.
Collectively, the studies reported on 1,126 dental implants, though two studies did not specify the implant count (Table 2). regarding administration routes, two studies used intravenous delivery, one did not specify, one used subcutaneous administration, and the remaining studies involved a mix of oral, intravenous, and subcutaneous routes (Table 3).
Patient success rates ranged from 38.9% to 100%, while dental implant success rates varied from 36.2% to 100%, with some studies omitting these data. notably, there was no substantial difference between patient and implant success rates.
Two studies specifically addressed MRONJ. In a case-control study by Giovannacci et al. [10], out of 15 patients, six experienced “implant surgery-triggered” MRONJ and nine were classified as “implant presence-triggered” MRONJ.
Risk of bias was evaluated using the cochrane RoB 2 tool for the RCT and the ROBINS-I tool for non-randomized studies. overall, one study was rated as serious, six as moderate, and one as low in risk of bias (Fig. 2).
Discussion
The objective of this systematic review was to assess the impact of antiresorptive therapy on dental implant success and failure rates. An analysis of 9 studies-including RCTs, cohort studies, case-control studies, and case series-that evaluated 1,126 dental implants provided robust insights into the relationship between antiresorptive drug use and implant outcomes.
Our findings reveal that dental implant success rates vary widely from 36.2% to 100%, with patient success rates ranging from 38.9% to 100%. This variability appears linked to the dosage and administration routes of antiresorptive drugs, primarily bisphosphonates and denosumab, with lower doses and oral administration correlating with higher success rates [11-17]. Conversely, a heightened risk of MRONJ was observed, especially when the presence or surgical placement of an implant exacerbated the condition [10,18-21].
The review underscores that low-dose, orally administered antiresorptive therapy tends to favor peri-implant bone resorption conducive to osseointegration, whereas high-dose or intravenous administration may increase the risk profile. these findings stress the need for meticulous pre-operative assessment and possible modifications in treatment protocols to mitigate MRONJ risks [22,23].
Comparison with previous research
Our results align with earlier studies [22-24], indicating that low doses of antiresorptive drugs lower implant failure risk. However, while some reviews reported a uniformly negative impact of high-dose therapy, our data suggest a more nuanced picture-success rates from 36.2% to 100% likely reflect differences in patient populations, drug regimens, and surgical protocols. The distinction between "implant surgery-triggered" and "implant presence-triggered" MRONJ [10,18]. adds further complexity, and the limited data on denosumab call for additional research [16].
Clinical implications
Clinically, these insights advocate for a personalized approach when planning dental implant procedures in patients undergoing antiresorptive therapy. a thorough evaluation of the drug type, dosage, and administration route is essential, as low-dose and oral routes are associated with reduced MRONJ risk and better implant outcomes, whereas high-dose intravenous administration requires a careful benefit-risk assessment [1,7]. Strategies such as enhanced screening, modified surgical techniques, and even drug holidays-despite ongoing debates [25-27]. may improve patient safety. multidisciplinary collaboration between dental surgeons and medical specialists is key to optimizing treatment protocols.
Methodological considerations
Adhering to PRISMA guidelines, our review integrated various study designs, which enriched the analysis but also introduced heterogeneity regarding patient populations, antiresorptive protocols, and follow-up durations. The focus on studies from the last decade, predominantly on bisphosphonates and denosumab, might have excluded earlier or broader data on other antiresorptive agents. Variability in reporting implant outcomes and MRONJ definitions further complicates direct comparisons.
Risk of bias assessment
Risk of bias was evaluated using the cochrane RoB 2 tool for RCTs and ROBINS-I for non-randomized studies. while the RCT showed a relatively low risk of bias, non-randomized studies exhibited a range from low to critical-mainly due to issues in confounding and participant selection. These biases necessitate cautious interpretation and highlight the need for more rigorous future studies.
Limitations of the review
Our review's limitations include the exclusion of alternative study designs, language restrictions to english, and a publication timeframe starting in 2013, potentially omitting foundational studies. moreover, variability in antiresorptive protocols and inconsistencies in outcome reporting across studies limit the generalizability of our findings. these factors underscore the need for standardized research to more clearly define the relationship between antiresorptive therapy and dental implant outcomes.
In conclusion, while low-dose, oral antiresorptive therapy appears relatively safe for dental implant procedures, high-dose regimens require careful assessment. our findings emphasize the importance of individualized treatment planning and further research to refine clinical guidelines for dental implantology in patients undergoing antiresorptive therapy.
Notes
Conflicts of Interest
None