Analysis of sterilization record management in dental clinic
Article information
Abstract
Purpose
This study aims to assess the current status of sterilization record management in dental clinics and propose measures to establish a systematic sterilization log management framework.
Materials and Methods
To examine the status of sterilization management, an online survey was conducted among members of the Korean Dental Association from May to June 2024. A total of 261 responses were analyzed. Frequency analysis was used to examine the general characteristics of respondents and the features of sterilization log creation and storage. Differences by type of medical institution were analyzed using the Chi-square test. Statistical significance was set at 0.05.
Results
Regarding sterilization log creation, 26.8% of respondents reported that they keep such records. Among them, 72.0% of dental hospitals and 22.0% of dental clinics reported doing so, with a statistically significant difference (p<0.05). For sterilization log management, 24.5% reported that they manage it, with dental hospitals at 72.0% and dental clinics at 19.5%, showing a statistically significant difference (p<0.05). Regarding sterilization log retention, the overall rate was 67.1%, with 83.3% for dental hospitals and 61.5% for dental clinics, also showing a statistically significant difference (p<0.05).
Conclusion
Sterilization logs hold significance beyond mere recordkeeping, serving as foundational data for quality management in healthcare and ensuring patient safety. The introduction of standardized forms, enhancement of governmental support systems, and implementation of integrated management strategies should be discussed at the policy level.
Introduction
Medical instruments and environments used in healthcare institutions can act as vectors for infection transmission; thus, proper disinfection and sterilization are critical [1]. In 2024, the Korea Disease Control and Prevention Agency (KDCA) announced the 2nd comprehensive plan for healthcare-associated infection control (2023-2027), aiming to minimize infection spread within medical institutions [2]. Notably, the plan expands infection control beyond acute care hospitals to include smaller hospitals, emphasizing the need to address areas with inadequate infection control systems and establish appropriate evaluation and compensation mechanisms. Before disinfection and sterilization, all used medical instruments must be cleaned, as organic materials on instruments can interfere with the sterilization process and potentially cause healthcare-associated infections [3,4] article 6 of the “Guidelines for disinfection of medical instruments and materials in medical Institutions” mandates the management of sterilization-related records, such as sterilization logs, routine inspections, maintenance logs, and recall records [5]. However, in dental practices, there are no specific standards or protocols for recording and storing such sterilization records. Moreover, there are no standardized formats or recommendations from the ministry of health and welfare, the korean dental association, or academic societies. As a result, each dental clinic maintains sterilization records based on its own environment, leading to missing or incomplete documentation and difficulties in record management. Administrative burdens related to processing and storing these records also contribute to infection control fatigue. Dental environments pose a high risk of infection due to aerosol-generating procedures, making infection prevention and control essential for both patients and healthcare workers [6,7]. Instruments used during dental procedures can serve as transmission routes for bacteria and viruses, necessitating stringent sterilization practices [8,9]. Proper sterilization ensures patient safety, protects healthcare workers, and ultimately enhances the credibility of dental services [10-12]. However, in dental clinics, managing sterilization-related administrative tasks is complicated due to the lack of clear guidelines, leading to increased workload and potential resource strain [13]. Therefore, establishing an efficient sterilization management system is essential. This study aims to investigate the status of sterilization record management in dental clinics and propose a management framework for sterilization logs based on the ministry of health and welfare's “dental infection control standard policy & procedure”.
Materials and Methods
To investigate the status of sterilization management, a survey was conducted among members of the Korean Dental Association. The participants were dentists who operate dental hospitals or clinics; public institutions and general hospitals or higher-level medical institutions were excluded. The sample size was calculated using G-Power, applying an effect size of 0.5, a power of 0.8, and a significance level (α) of 0.05, yielding a required sample of 260 participants. Taking into account potential dropouts, 280 responses were collected. After excluding 19 incomplete or insincere responses, a total of 261 were included in the final analysis.
The survey was conducted using an online survey platform (Naver form), and the survey period was from May to June 2024. The questionnaire was modified and supplemented to fit the purpose of this study by referring to the Standard Policy Manual for Dental Infection Control published by the Ministry of Health and Welfare, the CDC’s Infection Prevention Checklist, and the pilot report on the development of an infection control survey system for dental medical institutions by the Korea Disease Control and Prevention Agency. The questionnaire consisted of five items on general characteristics and eight items related to the preparation and management of sterilization logs. The general characteristics included type of medical institution, location, duration of operation, average number of patients per day (based on the past month), and the number of dental unit chairs. The sterilization log section included questions on whether a sterilization log is written, whether the log is supervised, the type of log format, the preferred type of log, items recorded in the log, whether the log is stored, the method of storage, and the storage period. The questions on writing the sterilization log and supervision of the log were answered with “Yes” or “No.” The type of format used for the sterilization log was answered with “Same format used” or “Different formats used.” The preferred log format was answered as either “Composed of essential items only” or “A detailed format that allows checking of specific contents.” Items recorded in the sterilization log (multiple responses allowed) included: type of sterilizer, date of sterilizer operation, number of operations, start and end time of operation, operator’s name, information on the load, Lot No., mechanical indicator confirmation, chemical indicator confirmation, biological indicator confirmation, supervisor confirmation, and “not recorded.” Storage of the sterilization log was answered with “Yes” or “No.” The method of storage was answered with “Paper document, electronic document, or not stored.” The retention period of the sterilization log was answered as either “Less than 3 years” or “3 years or more.”
Data analysis was performed using the Statistical Packages for the Social Sciences (SPSS) version 26.0 (SPSS Inc., NY, USA). Frequency analysis was conducted to examine the general characteristics of respondents and the features of sterilization log management. Chi-square tests were used to identify differences based on the type of medical institution. The level of statistical significance was set at p < 0.05.
Results
General characteristics of study participants
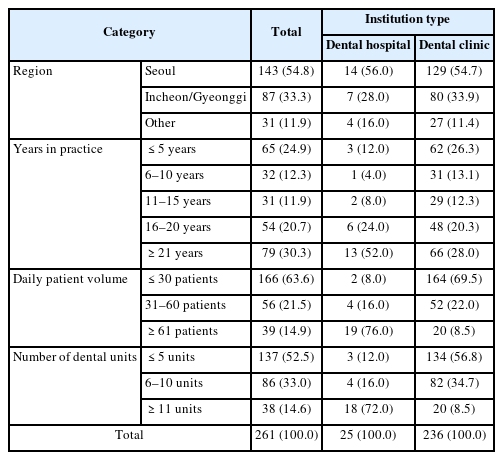
The general characteristics of the study participants are shown in Table 1. The region distribution was 54.8% in Seoul, 33.3% in Incheon/Gyeonggi, and 11.9% in other areas. Regarding the duration of practice, 30.3% had been in practice for over 21 years, and 24.9% for 5 years or less. In terms of patient volume, 63.6% treated 30 or fewer patients per day, and 52.5% had five or fewer dental units.
Sterilization log management
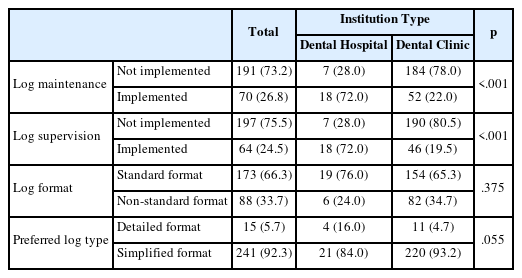
The results for sterilization log management are shown in Table 2. Approximately 26.8% of respondents reported maintaining sterilization logs. Among them, 72.0% were from dental hospitals, and 22.0% were from dental clinics, indicating a statistically significant difference (p<0.05). Regarding supervision of these logs, 24.5% reported having oversight, with 72.0% from dental hospitals and 19.5% from dental clinics, also showing a statistically significant difference (p<0.05). When asked about the format of the sterilization logs, 66.3% indicated using a standardized format. Specifically, 76.0% of dental hospitals and 65.3% of dental clinics used the same format, with no statistically significant difference (p>0.05). Regarding preferred log types, 92.3% favored simplified formats containing only essential items(p>0.05).
Sterilization log content
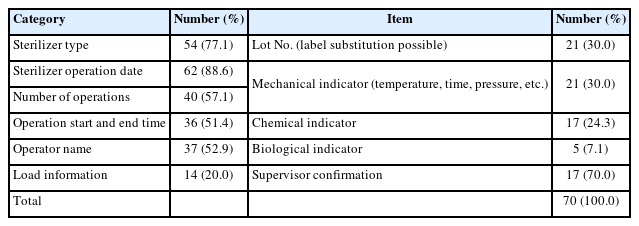
The contents of the sterilization log are presented in Table 3. The recommended essential items, as outlined in the Dental Infection Control Standard Policy Manual by the Ministry of Health and Welfare, include sterilizer type, date of operation, number of operations, start and end time of operation, operator name, load information, Lot number (can be substituted with a label), mechanical indicator confirmation (e.g., temperature, time, pressure), chemical indicator confirmation, biological indicator confirmation, and supervisor confirmation. The record rate for sterilizer type was 77.1%. The operation date was recorded in 88.6% of cases, and the number of operations in 57.1%. Start and end times of operations were documented in 51.4% of cases. The operator's name was recorded in 52.9% of cases. Load information was documented in 20.0% of cases, and lot numbers in 30.0%. Confirmation of mechanical indicators was recorded in 30.0% of cases, chemical indicators in 24.3%, and biological indicators in only 7.1%. Supervisor confirmation was recorded in 70.0% of cases.
Sterilization log retention
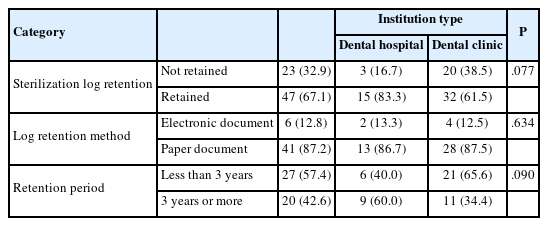
Sterilization log retention is shown in Table 4. According to the analysis, 67.1% of respondents reported keeping the log, with a higher implementation rate in dental hospitals (83.3%) compared to dental clinics (61.5%), which showed a statistically significant difference (p<0.05). Regarding the storage method, 87.2% retained the logs in paper form(p>0.05). As for the retention period, 57.4% stored them for less than 3 years(p>0.05).
Discussion
Medical institutions are required to manage sterilization-related records according to the "Guidelines for Disinfection of Medical Instruments and Supplies." However, in dental medical settings, there is a lack of specific standards for recording and storing sterilization-related tasks, resulting in management varying by institution without standardized guidelines. Therefore, this study was conducted to understand the current status of sterilization record management in private dental clinics and to provide basic data for establishing systematic sterilization log management methods.
The survey results showed that 26.8% of all respondents reported keeping sterilization logs, with dental hospitals at 72.0% and dental clinics at 22.0%, showing a significantly higher recording rate in dental hospitals (p<0.05). This is interpreted as being due to the structural difference that hospital-level institutions often have dedicated infection control personnel and established systematic infection control systems. According to the study by Jeong et al, the sterilization log recording rate in medical institutions was reported as 89.6%, noting differences in record-keeping and recall procedures depending on the number of hospitals beds [14]. In contrast, a study on infection control measures considering the characteristics of Korean dentistry reported a sterilization log recording rate of 61.4%, with tertiary hospitals, general hospitals, and dental university hospitals at 100%, certified dental institutions for infection control at 76.0%, and general dental clinics at 31.6%. Similar to prior research results [15], this study also showed a significantly lower recording and management rate in dental clinics compared to dental hospitals. This difference is interpreted as structural, reflecting the fact that hospital-level institutions often have dedicated infection control managers and established infection control systems, leading to differences in practice rates.
While selecting appropriate sterilization methods is important for the safe sterilization of medical instruments, confirming whether the sterilizer is functioning properly, maintaining sterilization logs, and having recall procedures in case of problems during sterilization are also necessary [14,16]. In this study, mechanical indicator records were 30.0%, chemical indicator records 24.3%, and biological indicator records 7.1%, showing low rates. The adequacy of sterilization is evaluated using mechanical, chemical, and biological indicators [17]. Mechanical indicators should be checked every time the sterilizer operates, but some hospitals did not perform these checks, consistent with prior research [14]. Biological indicator confirmation is known as the most accurate method to verify sterilizer performance, with recommended check frequencies being every cycle or daily for steam and E.O. gas sterilizers, and daily for plasma sterilizers [18], however, these recommendations were not consistently followed. Additionally, sterilization logs should be created and stored after sterilization and recall of sterilized items should occur if problems arise. However, this study found that sterilization logs were not consistently stored and recall procedures for already distributed items were not properly implemented. In particular, the finding that the confirmation rate using biological indicators is only 7.1% is considered a serious issue in terms of infection control. One of the main reasons for this low rate appears to be the perception in clinical settings that biological indicators are optional, despite being the most accurate method for verifying sterilization efficacy. Additionally, the financial burden associated with the use of biological indicators poses a significant barrier, especially for smaller institutions. Nevertheless, administrative and financial support systems that take these practical challenges into account remain notably insufficient. To address this issue, a multifaceted approach is required. First, efforts to raise awareness among dental healthcare providers regarding the importance of biological indicators are essential. At the same time, establishing a stronger institutional and regulatory framework, along with providing practical financial support, is necessary. Above all, it is crucial to create a structural environment that enables and encourages dental institutions to voluntarily enhance their infection control practices.
The method of sterilization log retention was predominantly paper-based at 87.2%, with electronic records at 12.8%. This trend is similar to previous nationwide surveys on central supply room operations(paper documents 73.1%, electronic 6.3%), indicating a continued reliance on manual records rather than digital systems. Furthermore, there was a significant difference between dental hospitals and dental clinics in the method of sterilization log retention, reflecting differences in infection control systems and resources between hospital and clinic levels.
The author suggests the introduction of standardized electronic sterilization logs to ensure accuracy and efficiency in infection control. Computerization of sterilization logs goes beyond simply digitizing record-keeping; it can greatly improve work efficiency by minimizing unnecessary manual entries. It also enhances accuracy and consistency of records, facilitates storage and management, and allows for long-term preservation. Importantly, electronically collected data can be analyzed and tracked, contributing to the establishment of a more systematic infection control system.
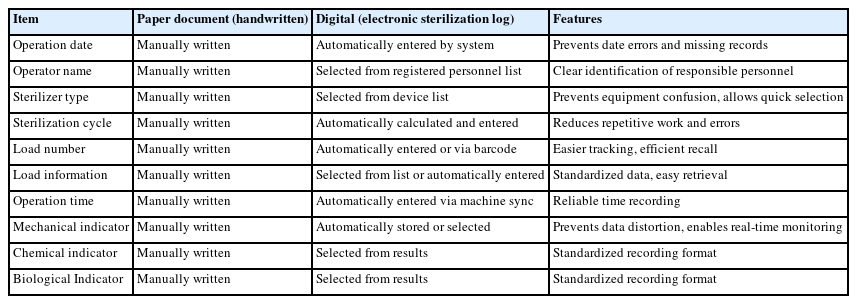
Table 5 compares the sterilization log recording items recommended by the Ministry of Health and Welfare’s standard manual between paper-based (manual) and computerized (electronic sterilization log) methods. This comparison confirms the superiority of computerized methods in terms of recording efficiency and accuracy.
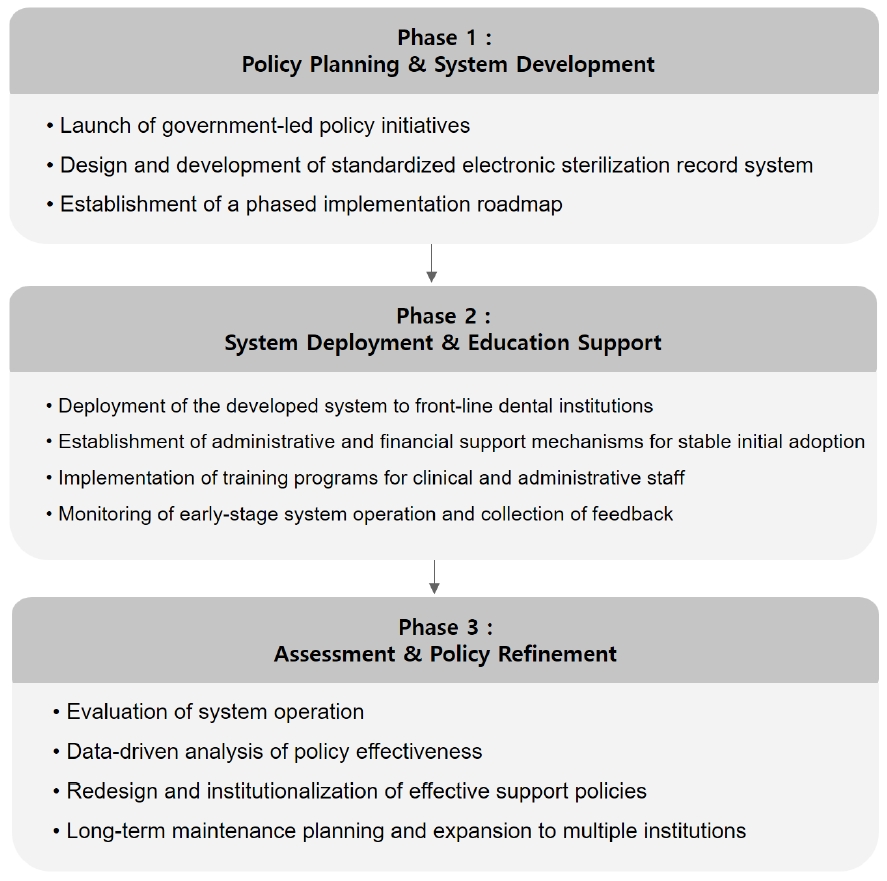
However, the implementation of a digital sterilization record system may involve technical and economic barriers. In particular, small-scale dental clinics may face practical limitations such as high initial costs and the need for staff training. Therefore, future technological developments should focus on enhancing accessibility through various complementary measures, such as lightweight cloud-based systems, user-friendly interface design, and minimal maintenance cost structures. Since such systems may be difficult for small clinics to adopt independently, support policies at the governmental and institutional levels will be essential to ensure widespread adoption. To effectively address these challenges, a phased national roadmap should be developed to ensure that related policies are implemented in a practical and sustainable manner. Initially, government-led policy initiatives should commission the development of standardized sterilization management systems, which can then be distributed to healthcare institutions along with comprehensive education and training programs to facilitate effective use. Subsequently, the administrative and financial burdens associated with sterilization management should be systematically assessed, and based on the findings, policy frameworks should be revised to establish a more robust and supportive system (Fig. 1).
This study conducted a survey targeting members of the Korean Dental Association. As a result, the sample may not sufficiently reflect various institutional characteristics such as the size, location, clinical departments, and level of infection control of dental institutions, thus limiting its representativeness. These factors may restrict the generalizability of the findings to all dental healthcare institutions. In addition, due to the nature of online surveys, it was difficult to control the respondents. There is a possibility that individuals with a higher interest in infection control were more likely to participate, introducing potential self-selection bias that may have influenced the results. Therefore, future research should aim to include dental institutions of various sizes and types and adopt a study design that comprehensively considers variables such as operational characteristics, infection control infrastructure, and staffing composition. Furthermore, survey methods that can minimize self-selection bias by encouraging participation from a broader range of respondents, regardless of their interest in infection control, should be considered. This would enable a more accurate and reliable analysis of the actual status of sterilization log recording and management.
In conclusion, this study has presented the necessity and potential of digital sterilization record systems. Continued discussion is needed to identify the specific technical and administrative conditions required for their effective implementation in clinical practice.
Notes
Conflicts of Interest
None